Molecular weight of NaOH or grams This compound is also known as Sodium Hydroxide. Use this page to learn how to convert between moles NaOH and gram.
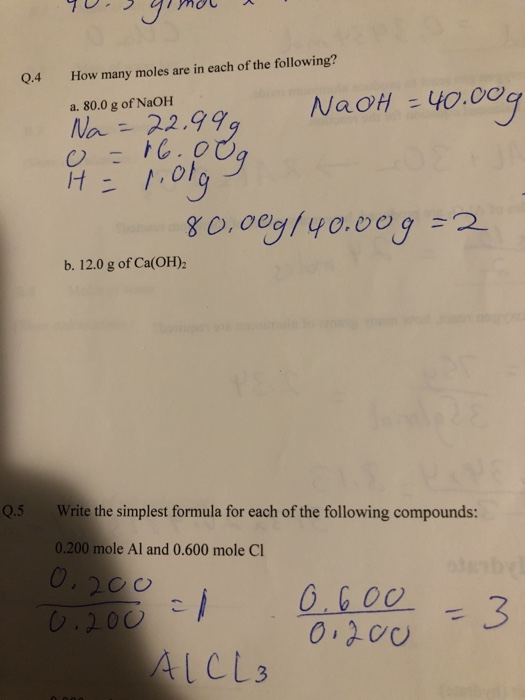
Solved 04 How Many Moles Are In Each Of The Following A Chegg Com
120 g of CaOH Q5 Write the simplest formula for each of the following compounds.

. One gram of NaOH is equal is to 000225 moles. Keeping this in view how many moles are in 20g of NaOH. 1 mole is equal to 1 moles NaOH or 3999711 grams.
Chemistry questions and answers. Subsequently question is how many moles are in 80g of NaOH. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg.
The answer is 3999711. You can view more details on each measurement unit. Hi I did the acid-base titration lab with HCl and 05M NaOH.
800 g of NaOH 229 NaOH-YOOO Na- b. The SI base unit for amount of substance is the mole. Find the molar mass of a water molecule H₂O.
Imagine you have 6 liters of pure water. 3999710928 gmole 40 gmole 60 g NaOH 60g 3999710928 gmole 150010841 moles 15 moles Please get a periodic table for this stuff. Which of the following statements.
Now weight of NaoH molecule 2316140. Molecular mass of NAOH is2316140 where 2316and1are the molecular masses of NAOAND H RESPECTIVELYTHUS REQUIRED NO OF MOLES GIVEN WEIGHTMOLECULAR weight8040moles. 80 gm of NaOH is two moles so you have two moles of sodium thus two times Avogadros constant of sodium atoms.
Therefore 80g is 200 moles 80 40. 0200 mole Al and 0600 mole Cl 0080 mole Ba 0080 mole S 0320 mole O When 250 g of copper Cu reacts with oxygen the copper oxide product has a mass. We assume you are converting between grams NaOH and mole.
How many grams are in 12 moles of he. This compound is also known as Sodium Hydroxide. 04 How many moles are in each of the following.
How many moles are there in 80 grams of Naoh. Sodium Na is 2299g Oxygen O is 1600g and Hydrogen H is 101g. November 11 2021 thanh.
Given mass in g find number of moles. Sodium Hydroxide NaOH FW. Just under 50g.
Divide the weight by the atomic or molecular mass. Take a look at the example of how to calculate moles from grams. The molar mass for NaOH is 4000g.
Molecular weight of NaOH or mol. How many grams is 10 moles of water. If the average human body discharges 925.
Dynamic Periodic Table Avogadro constant - Wikipedia. Then 80 gm of NaOH has 2 moles. Note that rounding errors may occur so always check the results.
So in 40 gm of NaOH has one mole. How many moles are in gram of NaOH. Molarity is defined as moles of solute which in your case is sodium hydroxide NaOH divided by liters of solution.
1 grams NaOH is equal to 0025001806380511 mole. Use a periodic table to find its atomic or molecular mass. 800 g of NaOH b.
0 g of CO2 per day. 0200 mole Al and 0600 mole Cl Оboo- 3 3. How many moles are in 800 g of NaOH.
Colorbluemolarity moles of soluteliters of solution SImply put a 1-M solution will have 1 mole of solute dissolved in 1 liter of solution. This expression means that 2 moles or 2 x 40 g 80 g of NaOH are dissolved in enough water to make one liter of solution. Now you know that your solution has a molarity of 0150 M and a volume of.
120 g of Ca OH2 5 Write the simplest formula for each of the following compounds. My English isnt good but is it asking me to find the moles of NaOH using the. Weight of Na 23.
Check your results with Omni Calculator. 80 40 2 mol. Number of moles which make up 20 grams of NaOH 20 grams 1 Mole 40 grams 05 moles.
Therefore if you have 8000g of NaOH there are two moles. Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³. The SI base unit for amount of substance is the mole.
I need to calculate the moles of NaOH from molarity of NaOH and the average volume used.
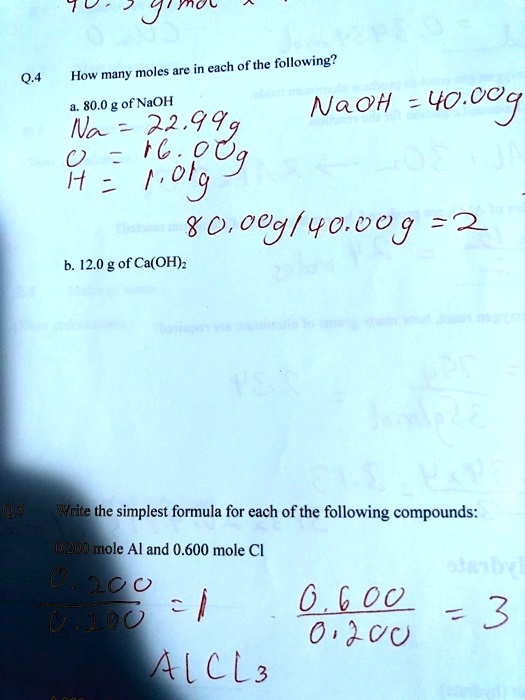
Solved 7 U Nou 6 04 How Many Moles Are In Each Ofthe Following 80 0 G Of Naoh Naoh 40 0o Na 29939 24 0 640 Bo0 0h 600 0 8 B 12 0 G Of


0 Comments